Key Takeaways
- Tirzepatide is FDA-approved as Mounjaro for diabetes and Zepbound for weight loss, while retatrutide is a triple agonist still in Phase 3 trials.
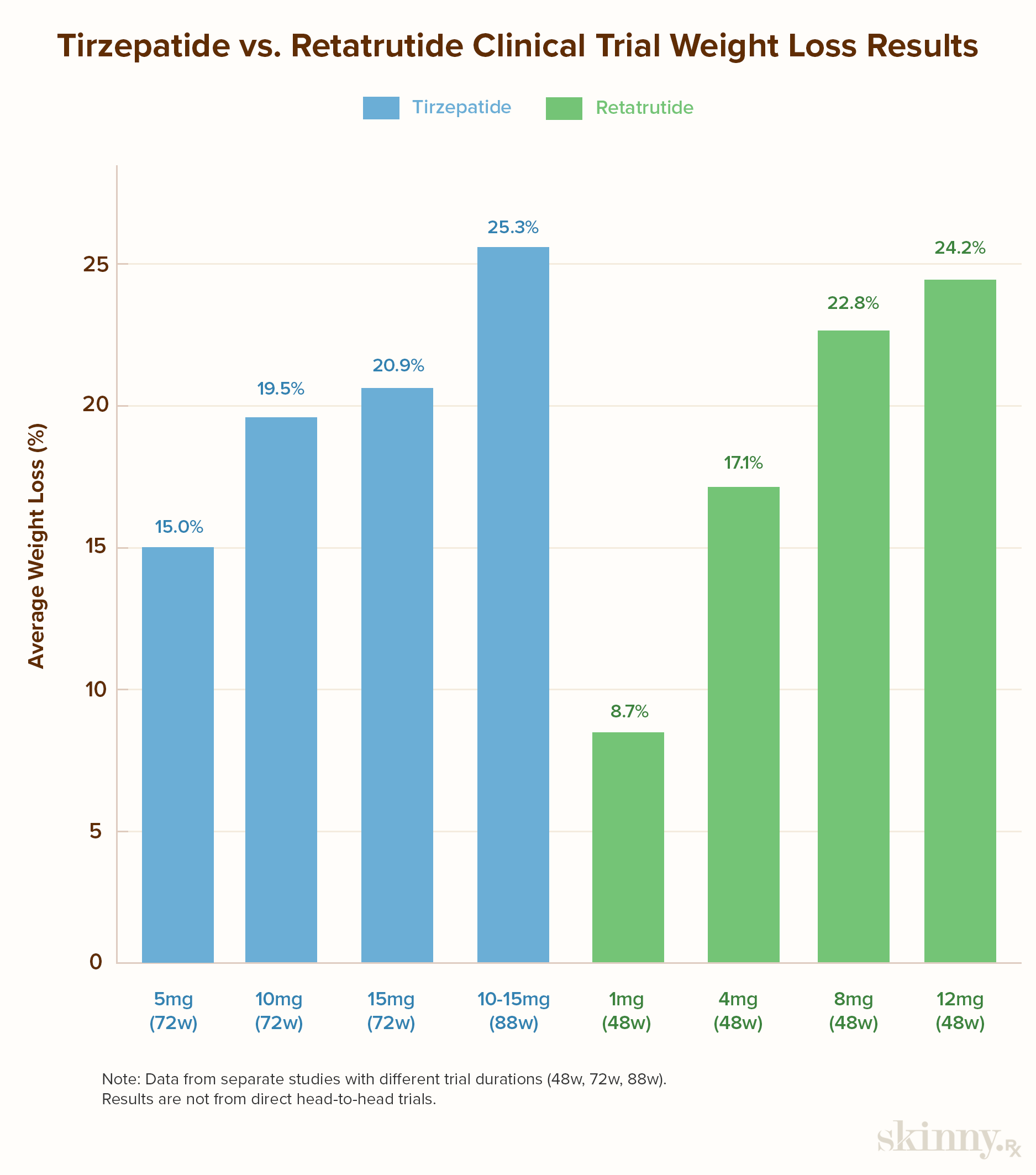
- Trials show tirzepatide up to 25% weight loss at 88 weeks; retatrutide reaches 24% at 48 weeks.
- Both cause GI side effects; Zepbound adds injection-site reactions and hair loss, while retatrutide studies note fatigue and abdominal pain.
- Tirzepatide is titrated weekly up to 15 mg; retatrutide has no established dosing.
- Tirzepatide costs over $1,000 monthly before insurance; retatrutide isn’t yet commercially available.
Conversations about weight loss drugs often center around Wegovy and Ozempic. But two newer drugs, tirzepatide and retatrutide, are attracting attention too. Tirzepatide has been available since 2022 as the brand-name Mounjaro and Zepbound and as a compounded medication. Retratrutide is still in clinical trials, but early trial results suggest it may provide another viable option.
Here’s what you need to know about these two medications, including how they both affect weight management and blood sugar control for type 2 diabetes.
Retatrutide vs. Tirzepatide: Understanding the Key Differences
What Are Retatrutide and Tirzepatide?
Both retatrutide and tirzepatide act like naturally occurring hormones. As a dual agonist, tirzepatide mimics two hormones:
- Glucose-dependent insulinotropic polypeptide (GIP): released earlier in a meal to help the body more effectively break down sugar from food.
- Glucagon-like peptide-1 (GLP-1): released later in a meal to regulate blood sugar and appetite. It also promotes feeling full for longer after a meal.
Retatrutide is a triple agonist, meaning it mimics three naturally occurring hormones, the two above (GIP and GLP-1) and glucagon. When used as part of this drug, the glucagon component enhances fat metabolism by breaking down stored fat (lipolysis) and boosts thermogenesis, which is your body's heat production process.
In practical terms, this means your body burns more calories, even when you’re resting. The increase in calories burned even when you’re at rest, combined with the drug’s appetite regulation effect, helps support weight loss and better blood sugar control.
While tirzepatide and retatrutide offer many of the same benefits, such as improved blood sugar control for those with type 2 diabetes and weight management, early studies suggest retatrutide may be better at treating diabetic kidney disease and promoting weight loss, in part because it mimics three hormones instead of two.
FDA Approved Uses
Tirzepatide comes under two brand names: Mounjaro and Zepbound. Mounjaro is FDA-approved for blood sugar control in adults with type 2 diabetes but is also sometimes used off-label for weight loss. Off-label uses are not FDA approved, meaning the FDA doesn’t guarantee their effectiveness or safety for the off-label purpose.
Zepbound is FDA-approved for weight loss in individuals with a BMI of 30 or higher or a BMI of 27 or higher with at least one weight-related condition, such as heart disease, sleep apnea, or type 2 diabetes.
There’s also compounded tirzepatide. Compounded medications are created in licensed labs that use active ingredients such as tirzepatide. They are not FDA-approved, meaning the amount or quality of the active and inactive ingredients isn’t guaranteed, but they are legal to purchase.
For tirzepatide specifically, compounded versions may also be available as dissolving tablets instead of an injection, which could be a good option for anyone who is needle-adverse.
Retatrutide is not yet approved by the FDA because it’s still in clinical trials. Before a drug is approved, it usually has to go through three trial phases. There is also a fourth trial phase for post-market surveillance. Retatrutide has gone through two of the four and is currently in phase three trials.

Ready to Explore Your Options?
Take a quick quiz to see if tirzepatide could be a fit for your weight loss goals and get guidance tailored to you.
Weight Loss Efficacy
When it comes to weight management, retatrutide will likely yield more results than tirzepatide. However, no completed studies compare these two drugs head on.
There are clinical trials underway to compare the two for weight loss in adults. Once these studies are complete, we may have a clearer understanding of tirzepatide vs retatrutide.
What we do have for now is the results from weight loss trials on each drug independently.
Tirzepatide
- In the SURMOUNT-1 trial, individuals taking a 5mg weekly dose experienced 15.0% weight loss over 72 weeks. Those taking a 10mg dose experienced a 19.5% reduction, while participants on a 15mg dose saw a 20.9% reduction.
- In the SURMOUNT-4 trial, the overall mean weight reduction was 25.3% in 88 weeks.
Retatrutide
- After 48 weeks, participants in a phase 2 trial experienced 8.7% weight reduction on a 1mg dose, 17.1% on a 4mg dose, 22.8% on a 8mg dose, and 24.2% on a 12mg dose.
- In a phase 2 trial looking at liver fat loss, healthy liver fat levels were achieved by 27% of participants on a 1mg dose, 52% on 4mg, 79% on 8mg, and 86% on 12 mg, compared to 0% of participants in the placebo group.
Side Effects of Each
As an FDA-approved drug, tirzepatide’s side effects are well-established. Common ones are:
- Abdominal pain
- Dizziness
- Sweating
- Confusion
- Headache
- Shakiness
- Fast heartbeat
- Mood changes
- Nausea
- Diarrhea
- Vomiting
- Decreased appetite
- Constipation
- Indigestion
Allergic reactions at the injection site and hair loss are also reported for Zepbound, but not Mounjaro.
Since retatrutide isn’t yet FDA-approved, there isn’t a drug label listing the side effects. Early studies suggest some people may experience:
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Fatigue
- Abdominal pain
Dosing and Administration
Tirzepatide usually comes as a weekly injection, though some compounded versions are available as tablets. Patients administer a weekly injection at home into the thigh, abdomen, or upper arm at any time of day, with or without a meal.
For both Mounjaro and Zepbound, titration is recommended, which is when you start on a lower dose and increase gradually. Both start at 2.5mg dose. If there are no severe side effects, someone can increase the dose to 5mg at the four week mark.
The maximum recommended dose for Mounjaro and Zepbound is 15mg a week, though not everyone requires this dose to see results. Work with a healthcare provider to figure out when it’s safe to increase the dosage and what is the best maximum dose for your needs.
Since retatrutide is in clinical trials, it is not yet available to the public. That means there are no guidelines on dosing and administration practices, though the drug is often administered in trials as a once-weekly injection.
Cost and Accessibility
Not to sound like a broken record, but since retatrutide is not yet available to the public, there are no established prices. The only way to safely get this drug is to enroll in a clinical trial, and the FDA has issued a warning to avoid compounded medications that claim to have retatrutide.
Tirzepatide is available to the public in three forms. All have a different list price, which is the cost before insurance or any discounts.
If using insurance, most plans cover FDA-approved medications, including Zepbound and Mounjaro. Usually, a medication must be prescribed for a use stated on the FDA label. A doctor will submit a request to the insurance provider, detailing the exact reason it’s medically necessary. The insurance company then accepts or denies. If denied, there’s a 30 day window to submit an appeal.
When covered by insurance, Eli Lilly, the manufacturer of both drugs, states that out-of-pocket costs for the consumer could be as low as $25 a month for Zepbound or Mounjaro.
Eli Lilly also claims that those who are self-paying (i.e. paying without insurance) could get Zepbound for as low as $349 (for a 2.5mg dose) if they purchase directly through the manufacturer’s pharmacy. There isn’t a similar savings program for Mounjaro.
Patient Selection: Who Is a Better Candidate for Each?
As of now, only tirzepatide is available, with Mounjaro being the FDA-approved option for blood sugar control for those with type 2 diabetes and Zepbound being the approved choice for weight management.
That said, retatrutide may be more effective at promoting weight loss. Some early research suggests it may even have a more favorable safety profile and possibly fewer side effects.
The Future of Incretin-Based Therapies
Retatrutide shows great promise but still has a ways to go until it’s commercially available. Here’s a quick look at retatrutide’s journey so far and what must happen before the FDA considers approving it.
Which Drug Comes Out on Top?
For those seeking a medication to help with blood sugar control or weight management, tirzepatide is the commercially available choice. Retatrutide will likely be more effective for weight management, but it’s still in clinical trials.
Weight loss medications have already made a big impact, and it’ll be exciting to see what retatrutide brings to the table once commercially available.

Ready to Explore Your Options?
Take a quick quiz to see if tirzepatide could be a fit for your weight loss goals and get guidance tailored to you.


 Medically Reviewed
Medically Reviewed